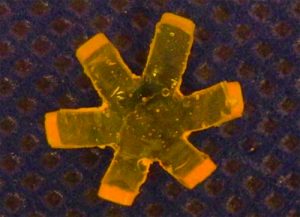
Using this idea, scientists could make a surgical stent, for example. Start out with a narrow tube. Then raise it to 98.6 degrees—the temperature of the human body—and bend it into a round shape to shore up the walls of an artery. Bring it back down to room temperature, and it’s a tube again.
Because the stent starts out as a narrow tube, a surgeon could insert it in a minimally invasive way. Then the patient’s body heat would bring it back to the stent shape once it was inside.
http://vimeo.com/104336981
The U.S. military is interested in shape-shifting, Sheiko says, for all kinds of reasons. Sheiko grabs a magazine off his desk. “Imagine I can put a piece of paper under your door and increase the temperature or shine some light on it, and it assembles into some kind of a device,” he says.
Or imagine a plane with shape-shifting wings. “If you look at birds like hawks or eagles, they change the geometry of their wings,” Sheiko says. “If they want to fly and look for prey, their wings have one geometry, but the geometry changes when they attack.”
And imagine if all these complex shape transformations were trigged remotely. Instead of using temperature, the lab has also used certain wavelengths of sound, magnets, and light waves to trigger a material to change.
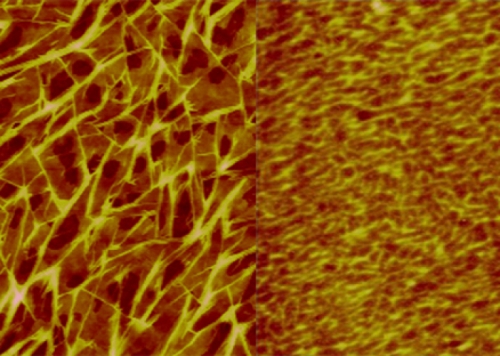
Sheiko thinks that soft-matter science—the study of polymers and other soft materials like liquid crystals, gels, and foams—will be the nuclear physics of the twenty-first century: a new frontier of scientific discovery. There are fundamental principles we don’t yet understand about how molecules such as polymers self-organize and are held together by weak forces to work in a system.
“The ultimate example of this,” Sheiko says, “is the human body.”